Groundbreaking research led by scientists at the Karl Landsteiner University of Health Sciences sheds light on how α2δ protein mutations affect neurodevelopmental processes.
Krems, Austria, 29. January 2025 – A recent study has uncovered how specific genetic mutations in α2δ-1 and α2δ-3 proteins linked to autism spectrum disorders (ASD) alter neuronal functionality. These mutations significantly reduce the proteins’ membrane expression and synaptic targeting but do not impair calcium channel activity or trans-synaptic signalling. Conducted at Karl Landsteiner University of Health Sciences (KL Krems) within the research focus Mental Health and Neuroscience, the research provides a fresh perspective on how subtle disruptions in protein function may influence synapse formation and neuronal networks. The results underscore the need for new experimental tools and might offer new angles for developing targeted treatments addressing the complex biology of ASD.
Autism spectrum disorder, a complex neurodevelopmental condition, affects millions worldwide and is marked by challenges in communication, social behaviour, and repetitive actions. A significant proportion of ASD cases are linked to genetic factors, with mutations in the CACNA2D1 and CACNA2D3 genes — which encode α2δ-1 and α2δ-3 proteins—emerging as critical players. These proteins regulate calcium channels, synapse formation, and neuronal connectivity, yet their exact role in ASD has remained elusive. To bridge this gap, KL Krems’ Division of Physiology embarked on a comprehensive study to explore cellular pathophysiological mechanisms of mutations in these genes.
A Subtle Disruption
„These findings redefine how we understand the role of α2δ proteins in brain development”, says Prof. Dr. Gerald Obermair, Head of the Division of Physiology at KL Krems. His team revealed that two specific mutations — p.R351T in α2δ-1 and p.A275T in α2δ-3 — reduce the presence of these proteins at neuronal membranes, thereby disrupting the synaptic localization. „What makes this discovery particularly compelling is that while the mutations don’t affect classical calcium channel functions, subtle changes may significantly affect synaptic functions”, Sabrin Haddad, M.Sc., first author of the publication and PhD student in the team of Prof. Obermair, adds.
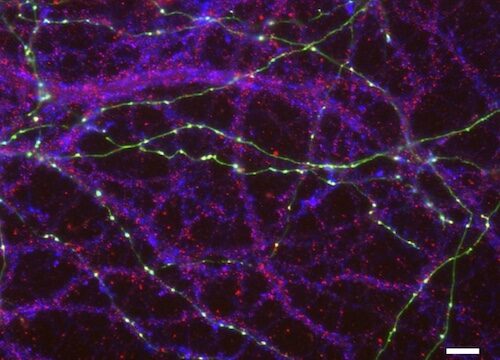
The research utilized cultured hippocampal neurons and advanced electrophysiological methods to assess how these mutations impact neuronal processes. The results showed that both p.R351T and p.A275T mutations led to a reduction in the membrane expression of α2δ proteins, particularly in dendrites and axons, the critical sites of neuronal connectivity.
Interestingly, the p.A275T mutation in α2δ-3 was also found to alter the protein’s glycosylation — a process critical for maintaining protein stability and function. Despite these structural disruptions, calcium channel activity and synaptic signalling were unaffected, indicating that the mutations’ impact is likely on the architecture of synapses rather than their signalling properties.
Implications for the Future
The study confirmed that the overall levels of α2δ proteins remained stable, suggesting that the mutations primarily influence their structural and surface localization roles within neurons. These findings shift the focus from traditional views of calcium channel dysfunction to exploring how protein mislocalization might affect neuronal networks. „Our work shows that the effects of these mutations are nuanced, underscoring the need for deeper investigations into their role in neurodevelopmental disorders like autism”, Prof. Obermair states.
This research adds a critical piece to the puzzle of autism’s complex genetic underpinnings. By revealing alternative pathways through which genetic mutations affect brain development, the study sets the stage for innovative experimental approaches as well as offering a new perspective on options for novel therapeutic options.
Original publication: Autism-Linked Mutations in α2δ-1 and α2δ-3 Reduce Protein Membrane Expression but Affect Neither Calcium Channels nor Trans-Synaptic Signaling. S. Haddad, M. Campiglio, M. Hessenberger, C. Ablinger, C. Eibl & G. J. Obermair. pharmaceuticals 2024, 17, 1608. DOI: 10.3390/ph17121608. https://kris.kl.ac.at/de/publications/autism-linked-mutations-in-%CE%B12%CE%B4-1-and-%CE%B12%CE%B4-3-reduce-protein-membran.
Images available on request
Karl Landsteiner University of Health Sciences (01/2025)
The Karl Landsteiner University of Health Sciences (KL Krems) is an educational and research institution on the Campus Krems and recognised throughout Europe. KL Krems offers modern, demand-oriented education and continuing education in medicine and psychology as well as a PhD programme in Mental Health and Neuroscience. The flexible educational programme is tailored to the needs of students, the requirements of the labour market and the challenges of science. The three university hospitals in Krems, St. Pölten and Tulln and the MedAustron Ion Therapy and Research Centre in Wiener Neustadt guarantee clinical teaching and research of the highest quality. In its research, KL Krems focuses on interdisciplinary fields with high relevance to health policy – including biomechanics, molecular oncology, mental health and neuroscience as well as the topic of water quality and the associated health aspects. KL Krems was founded in 2013 and accredited by the Austrian Agency for Quality Assurance and Accreditation (AQ Austria). https://www.kl.ac.at/en
Scientific Contact
Prof. Dr. Gerald Obermair
Division of Physiology
Department of Pharmacology, Physiology and Microbiology
Karl Landsteiner University of Health Sciences
Dr.-Karl-Dorrek-Straße 30
3500 Krems / Austria
T +43 2732 9004 12141
W https://www.kl.ac.at/
Karl Landsteiner University of Health Sciences
Mag. Selma Vrazalica, BA
Communications, PR & Marketing
Dr.-Karl-Dorrek-Straße 30
3500 Krems / Austria
T +43 2732 72090 237
M +43 664 883 99 603
Copy Editing & Distribution
PR&D – Public Relations for Research & Education
Dr. Barbara Bauder-Jelitto
Kollersteig 68
3400 Klosterneuburg / Austria
M +43 664 1576 350
L https://www.linkedin.com/company/prd-public-relations-für-forschung-bildung